PARDON THE INTERUPTION - OUR WEBSITE RESEARCH PAGE WILL BE UPDATED SOON!
RESEARCH
The ADNP Kids Research Foundation is funding science to understand the biology of ADNP and identify innovative therapeutic strategies and a cure for all individuals around the world with ADNP syndrome.
- NOTE: We are a 100% volunteer-ran patient organization, we do not pay university overhead fees as part of our grants or sponsorship research projects. If you are interested in discussing a grant opportunity please email [email protected].
|
OUR RESEARCH PLAN
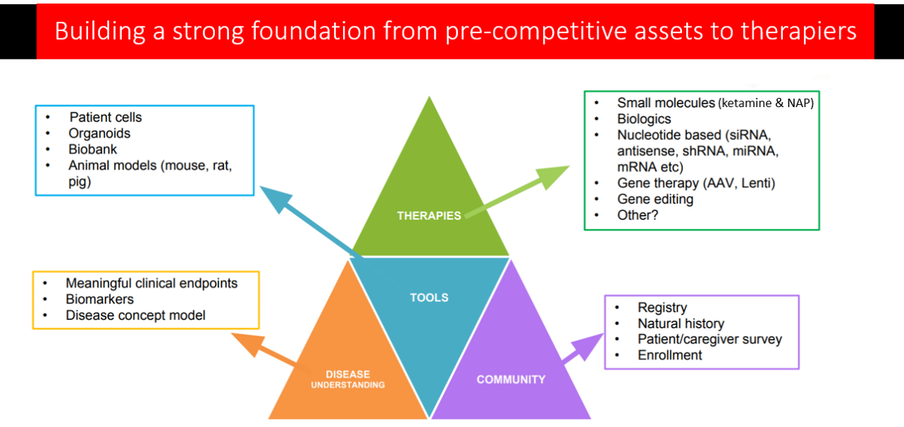
The ADNP Kids Research Foundation is funding and collaborating with organizations who are running research projects and clinical trials, testing both existing drugs, as well as treatments - by way of; high throughput screening, gene therapies and antisense oligonucleotide therapies. Information about current projects can be found below and will be updated periodically. We are moving forward in building our ADNP ASSETS, including a patient registry, biorepository, creation and characterization of IPC lines and ADNP animal models (3) that replicate the ADNP gene mutations that exist in our children. Current models produced at Universities are not easily shared and often come with a licensing agreement and fees that turn off potential researchers and biotech, so as we build our ASSETS and have them in accessible biorepositories, we put ourselves in a better position to attract biotech and pharma. The ASSETS created by the ADNP Kids Research Foundation will be cataloged and made easily available to quality researchers and biotech companies around the world to work on ADNP drug discovery and development with no restrictions or time consuming road blocks. |
CURRENT RESEARCH PROJECTS
DRUG TRIAL
LOW DOSE KETAMINE FOR ADNP
BIOMARKERS AND OUTCOME MEASURES
SENSORY REACTIVITY
Individuals with ADNP syndrome can be placed in one of two groups, with differing epigenetic signatures (which are reproducible DNA methylation changes).
SEAVER CENTER PROJECTS
ADNP STUDY & CENTER OF EXCELLENCE NEURODEVELOPMENTAL AND AUTISM
The SEAVER team has numerous collaborative projects as well:
DRUG TRIAL
LOW DOSE KETAMINE FOR ADNP
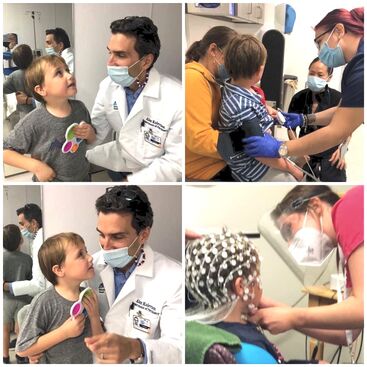
- Our ADNP research program partnership with the Seaver Autism Center at Mount Sinai (SEAVER) team overseen by Dr. Joseph Buxbaum has made huge strides. The most exciting project progress has been made by Dr. Alexander Kolevzon and team who completed the first-ever clinical trial for ADNP syndrome, testing low-dose ketamine as a potential treatment. In this FDA Phase 2A, single dose, open label study, ten children received intravenous ketamine and were monitored over the course of four weeks. Based on data from clinician-rated assessments and parent reports, they found ketamine to be associated with improvements in social behavior, attention deficit and hyperactivity, restricted and repetitive behaviors, and sensory sensitivities. Most importantly for this study was to prove safety to the FDA so that we may move to the next phase of our drug trial.
- Phase 2b: The original plan for ketamine was straightforward and cost effective. Since ketamine is a reopposed drug and the foundation holds IP, we initially hoped it unnecessary to conduct a Phase 3 FDA registration trial, which typically costs millions of dollars. Our primary objective was to do a placebo-controlled study to demonstrate efficacy and facilitate "off label" use. Given that ketamine is commercially available in generic form and relativity inexpensive, ketamine in its currently available state could potentially eliminate the need for insurance reimbursement altogether, and if written off label, would not require a Phase 3 FDA drug trial which would cost millions of dollars.
- The original plan was to run a placebo controlled crossover multi-site study enrolling 30 children within the same age range and criteria as our Phase 2a safety study. Once baseline and safety was monitored and parents where trained, we intended to have parents continue the trial dosing at home with a very well thought-out virtual follow schedule with the research team. However, due to ketamine's class, the FDA is requiring that drug administration must be 'clinically supervised', either 'in the clinic' or 'at-home by a nurse', each dose, which is approximated to be twice a week.
- To continue with in clinic or at-home dosing with a nurse to monitor would effectively double the cost of the trial and unfortunately was well beyond what the Foundation had available in current funds. Given this situation, we began looking into other ways to fund this project, such as supporting a very large grant application that Mount Sinai has submitted to the National Institutes of Health (NIH).
- Currently, the Team at Mount Sinai has a protocol approved by the FDA for in-clinic dosing and a grant that was very well received by the NIH with final decisions being made on funding in September 2023.
- The in-clinic dosing will occur twice weekly for 8 weeks and unfortunately place significant burden on families. The Foundation is exploring either dedicating funds to families to assist with travel, or raising more money to hire a nursing service to monitor participants for at-home drug administration.
- Additionally, because the foundation owns the patent(s), we are actively seeking a pharmaceutical partner to license ketamine. Although this will result in the treatment cost being set by a pharmaceutical company, it offers the potential for significant progress because pharmaceutical companies can run the drug trial and cover those expenses much faster than we can. If the treatment proves to be efficacious and successful through a Phase 3 drug trial with FDA approval, it opens the door for insurance reimbursement. This path represents a viable option for continuing the research and providing a potential treatment solution for patients with ADNP syndrome.
BIOMARKERS AND OUTCOME MEASURES
SENSORY REACTIVITY
- In clinical research, Dr. Paige Siper and team at SEAVER carried out detailed phenotyping of 22 individuals seen through an ADNP study with ADNP syndrome and published their observations that ADNP is associated with sensory reactivity symptoms, which may represent a therapeutic target.
- Event-related potentials, visual and auditory evoked potentials, and eye tracking tasks have shown distinctive changes to patients with ADNP syndrome. Electrophysiological tasks involve placing non-invasive electrodes on your child’s head. Your child will be asked to look at patterns on computer monitors and listen to different sounds.
Individuals with ADNP syndrome can be placed in one of two groups, with differing epigenetic signatures (which are reproducible DNA methylation changes).
SEAVER CENTER PROJECTS
ADNP STUDY & CENTER OF EXCELLENCE NEURODEVELOPMENTAL AND AUTISM
- The Seaver Autism Center at Mount Sinai Icahn School of Medicine in New York is the worlds only center for ADNP patients.
- The ADNP syndrome study launched in 2018 is ongoing, to understand the biology of the disorder
- Drug trail hub for the ADNP Kids Research Foundation sponsored drug trial.
The SEAVER team has numerous collaborative projects as well:
- In collaboration with Neucyte, they are working to characterize several patient-derived cell lines and hopefully develop additional assays for pharmacological testing.
- In collaboration with Scripps Institute, that have used a patient-derived cell line to screen a compound library (including thousands of possible small molecule drugs) in a high-throughput assay. They are now in the process of validating initial hits in the assays developed by the preclinical team and are in the process of publishing.
- In collaboration with Rumi Scientific, they have been able to characterize genetically engineered ADNP-deficient lines in their proprietary brain organoid platform and the next step is to confirm the findings in patient-derived cell lines.
|
SPONSORED STUDIES AND PROGRAMS
GENE THERAPY, ASO & CRISPRa-BASED GENE ACTIVATION THERAPY Rapid development and evaluation of multiple "best-in-class" gene therapy and "best-in-disease" (CRISPR Cas 9 epigenetic activation) treatments for ADNP syndrome delivered by viral vector, to increase ADNP expression. These approaches, directed by complementary leaders in their fields at both UC Davis and the MIND Institute, proposes the highest likelihood of successful therapeutic development. Proposal is a 3-year project costing approximately $4.3 million dollars. |
NET STUDY
Our first grant of 2021 was awarded to Dr. Thomas Frazier (CSO of Autism Speaks and Professor of Psychology at John Carrol University). Dr. Frazier is running the Neurobehavioral Evaluation Tool (NET) Study for autism and related genetic syndromes, which will include the participation of ADNP patients and their caregivers in the development and application of the tool. The grant is a joint collaborative effort with several other rare disease organization, primarily funded by PTEN Research, as well as Autism Speaks, SynGAP Research Fund and Malan Syndrome Foundation. The foundation has also connected Dr. Alex Kolevzon of the Seaver Autism Center to the project. His deep clinical expertise with ADNP patients will help inform the work of the NET project. *CURRENTLY RECRUITING PATIENTS FOR STUDY
FUNCTION
Our most recent and very exciting project is FUNCTION at RAREBASE. This is an innovative project involving "15 other similar disease patient organizations" working together on translational therapeutic discovery and development. The project aims to identify candidate drugs and targets based on a combination of high-throughput screening, stem cell biology, and next generation sequencing. The three distinct phases to Function: (1) Prediction Algorithm: Identify candidate drugs or drug targets based on gene expression. (2) Validation Engine: Validate Function drug predictions in patient-derived cell models. (3) Machine Learning: Leverage artificial intelligence and machine learning methods to continually improve prediction algorithms based on data from validation experiments. Initially, Function will screen a library of 2,500+ FDA approved drugs and a proprietary library of drug-like small molecules. In the future, Function will expand to novel compounds that cover a broad range of chemical structures.
Our first grant of 2021 was awarded to Dr. Thomas Frazier (CSO of Autism Speaks and Professor of Psychology at John Carrol University). Dr. Frazier is running the Neurobehavioral Evaluation Tool (NET) Study for autism and related genetic syndromes, which will include the participation of ADNP patients and their caregivers in the development and application of the tool. The grant is a joint collaborative effort with several other rare disease organization, primarily funded by PTEN Research, as well as Autism Speaks, SynGAP Research Fund and Malan Syndrome Foundation. The foundation has also connected Dr. Alex Kolevzon of the Seaver Autism Center to the project. His deep clinical expertise with ADNP patients will help inform the work of the NET project. *CURRENTLY RECRUITING PATIENTS FOR STUDY
FUNCTION
Our most recent and very exciting project is FUNCTION at RAREBASE. This is an innovative project involving "15 other similar disease patient organizations" working together on translational therapeutic discovery and development. The project aims to identify candidate drugs and targets based on a combination of high-throughput screening, stem cell biology, and next generation sequencing. The three distinct phases to Function: (1) Prediction Algorithm: Identify candidate drugs or drug targets based on gene expression. (2) Validation Engine: Validate Function drug predictions in patient-derived cell models. (3) Machine Learning: Leverage artificial intelligence and machine learning methods to continually improve prediction algorithms based on data from validation experiments. Initially, Function will screen a library of 2,500+ FDA approved drugs and a proprietary library of drug-like small molecules. In the future, Function will expand to novel compounds that cover a broad range of chemical structures.
ASSET DEVELOPMENT
MICE
We are developing 3 mice lines. Animal models are necessary to test new targeted drugs and gene therapy before in human testing can be done.
IPC LINES
The preclinical team led by Dr. Ana Kostic at SEAVER has developed genetically engineered and patient-derived stem cells using the blood from our children to identify deficiencies in nerve/brain cell activity due to ADNP mutations. They are examining whether ADNP mutations change the structure and function of nerve cells, compared to control cell lines. Any alterations in nerve cells caused by mutations in ADNP provide a means of identifying pathways and mechanisms that could be targeted pharmacologically to alleviate the symptoms of ADNP syndrome.
MICE
We are developing 3 mice lines. Animal models are necessary to test new targeted drugs and gene therapy before in human testing can be done.
IPC LINES
The preclinical team led by Dr. Ana Kostic at SEAVER has developed genetically engineered and patient-derived stem cells using the blood from our children to identify deficiencies in nerve/brain cell activity due to ADNP mutations. They are examining whether ADNP mutations change the structure and function of nerve cells, compared to control cell lines. Any alterations in nerve cells caused by mutations in ADNP provide a means of identifying pathways and mechanisms that could be targeted pharmacologically to alleviate the symptoms of ADNP syndrome.
- The ADNP Kids Research Foundation in collaboration with Combined Brain, are developing 6 patient derived IPC lines as well as 2 sibling derived IPC lines.
|
|
ADNP INTERNATIONAL PATIENT REGISTRY & NATURAL HISTORY STUDY:
Our Patient Registry has been IRB approved and is currently being launched. This registry will allow open access to deidentified information for research and the first ADNP Syndrome Natural History Study which is a critical component of the drug development and trial process. BIOBANK REPOSITORIES
|
PROOF OF CONCEPT STUDY
As we build awareness of ADNP Syndrome, researchers are now coming to ADNP Kids Research Foundation with proposals for innovative and impressive therapeutic projects. The projects listed below are ones that the foundation agrees are important and could be very impactful and are working with developers to move forward.
As we build awareness of ADNP Syndrome, researchers are now coming to ADNP Kids Research Foundation with proposals for innovative and impressive therapeutic projects. The projects listed below are ones that the foundation agrees are important and could be very impactful and are working with developers to move forward.
- ANTISENSE OLIGONUCLEOTIDE BASED THERAPY: Preliminary investigation of possible ASO therapeutic approach - project and company are CONFIDENTIAL
Our research is not possible without the support from families like you and the philanthropy from generous donors to fund our projects.
Together we can continue to make strides to increase the quality of life for individuals with ADNP syndrome.
If you would like to support our work on ADNP syndrome, please DONATE here.
Together we can continue to make strides to increase the quality of life for individuals with ADNP syndrome.
If you would like to support our work on ADNP syndrome, please DONATE here.